PMTA
What is a PMTA?
A Premarket Tobacco Product Application (PMTA) is an application that must be reviewed and approved by the Food and Drug Administration before a new tobacco product can be legally marketed in the United States. A PMTA must provide scientific data that demonstrates a product is appropriate for the protection of public health.
Vaping manufacturers including 21st Century Smoke® had until September 9, 2020, to submit a PMTA in order for their e-liquids products to remain legal.
What’s our status?
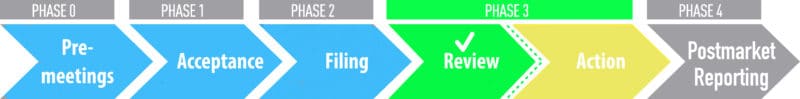
We are happy to announce our PMTA for 21st Century Smoke® products has been accepted and filed. Now it goes before Formal Scientific Review, the final step in the PMTA process. Once a marketing order is granted, the FDA will authorize 21st Century Smoke® products to stay on the market moving forward. YOU are the most important part of our business, therefore 21st Century Smoke® is ensuring we keep our brand compliant and available to all our great customers! Our PMTA progress will be updated here.